Benefits of Copper – In Skincare
Copper Required in the Human Body and in Skin
Copper is one of the “trace metals” required in small amounts for proper functioning of the human body and its various macro and microsystems, including enzyme systems. As such, there are minimum dietary requirements that must be met. These requirements are small in absolute amount of copper but if deficient amounts of copper are ingested, many systems fail to work properly.
For skin, copper is perhaps best known as a required cofactor in collagen synthesis. In addition, copper is a necessary metal in a number of other biochemical reactions occurring in skin, as listed below. [1. Biochemical, Physiological, and Molecular Aspects of Human Nutrition. Stipanuk MH and Caudill MA, eds. “Zinc, Copper, and Manganese. Grider A. 2013. Elsevier: USA. p830.]
Copper-Containing Enzyme — vs. — Enzymatic Function
Superoxide Dismutase — Antioxidant (superoxide degradation)
Lysyl Oxidase — Collagen, Elastin synthesis
Collagen Proline Dioxygenase — Collagen synthesis
Cytochrome Oxidase — Energy production
Tyrosinase — Melanin formation
Topical Applications for Copper in Wound Healing
There numerous potential applications for using copper in aesthetics. The medical literature details uses of copper ions as antimicrobials with potential to combat a variety of potential infectious processes including bacteria[2. Additivity and synergy between an antimicrobial peptide and inhibitors ions. Walkenhorst WF, Sundrud JN, Laviolette JM. Biochim Biophys Acta. 2014 Sep. 1839(9):2234-42. Epub 2014 May 17.], Herpes viruses [3. Efficacy and tolerability assessment of a topical formulation containing copper sulfate and hypericum perforatum on patients with herpes skin lesions: a comparative, randomized controlled trial. Dlewell A, Barnes M, Endres JR, Ahmed M, Ghambeer DK. J Drugs Dermatol. 2012 Feb. 11(2):209-15.], leishmaniasis[4. Antileishmanial activity of disulfiram and thiuram disulfide analogs in an ex vivo model system is selectively enhanced by the addition of divalent metal ions. Peniche AG, Renslo AR, Melby PC, Travi BL. Antimicrob Agents Chemother. 2015 Aug 3. Epub ahead of print.], and other conditions where infectious processes are implicated such as acne with Propionibacterium acnes[5. Pilot study of topical copper chlorophyllin complex in subjects with facial acne and large pores. Stephens TJ, McCook JP, Herndon JH Jr. J Drugs Dermatol. 2015 Jun. 14(6):589-92.]. Copper metal ions have been found in higher concentration around healing wounds and thus are implicated in wound healing and inflammatory processes[6. Zinc, copper, and selenium tissue levels and their relation to subcutaneous abscess, minor surgery, and wound healing in humans. Miratschijski U, Martin A, Jorgensen LN, Sampson B, Agren MS. Biol Trace Elem Res. 2013 Jun. 153(1-3):76-83. Epub 2013 Apr 18.]. The topical application of copper ion-containing ointments has been associated with improved wound healing[7. Rat epigastric flap survival and VEGF expression after local copper application. Frangoulis M, Georgiou P, Chrisostomidis C, Perrea D, Dontas I, Kavantzas N, Kostakis A, Papadopoulos O. Plast Reconstr Surg. 2007 Mar. 119(3):837-43.].
Proper Formulation Required – Copper may exist in a metallic form or an ionic form. The metallic form of copper is the type the public usually thinks of when visualizing this metal – even though copper in metallic form cannot be used by biologic systems. In order for humans and other organisms to benefit from copper, it must be present in ionic form. Ionic copper can be joined to enzyme systems and is the only form that is active in the human body. Ionic forms of copper look very different from copper metal. These have no “metallic” appearance because the copper in them is chemically bound to other substances.
Potentiating Effects of Copper on Collagen Synthesis
Collagen is the most prevalent protein in the body and most collagen is found in skin. Collagen serves as the structural framework for numerous tissues including skin, bone, teeth, tendons, and all other connective tissues. As well as adding copper to topical formulations designed to support collagen synthesis, other actives may also be added such as Vitamin C and growth factors.
Advances in formulation technology have now made it possible to combine copper with Vitamin C for topical use. The “second generation” Vitamin Cs, such as ADVANCE+™ Vitamin C and tetrahexyldecyl ascorbate, overcame limitations of the first generation of Vitamin C technology. In the past, combining copper and Vitamin C together resulted in a chemical reaction between the two substances causing a decrease in antioxidant activity. Since both Vitamin C and copper are necessary for the formation of healthy collagen, combining both ingredients in a single formula potentiates collagen formation. The second generation Vitamin Cs were a tremendous formulating advance allowing the combination of Vitamin C and copper with potentiation of the activity on collagen synthesis.
Growth factors such as Copper Tripeptide-1 also increase collagen synthesis.[8. Stimulation of collagen synthesis in fibroblast cultures by the tripeptide-copper complex glycyl-L-histidyl-L-lysine-Cu2+. Maquart FX, Pickart L, Laurent M, Gillery P, Monboisse JC, Borel JP. FEBS Lett. 1988 Oct 10. 238(2):343-6.] This growth factor is a tripeptide composed of the three amino acids glycine, histidine, and lysine. This natural molecule that is found in human skin and other tissues mediates its effect of encouraging collagen synthesis via decorin[9. The regulated synthesis of versican, decorin, and biglycan: extracellular matrix proteoglycans that influence cellular phenotype. Kinsella MG, Bressler SL, Wight TN. Crit Rev Eukaryot Gene Expr. 2004. 14(3):203-34.], a molecule intimately involved with the architecturally correct synthesis of collagen. Copper Tripeptide-1 also affects matrix metalloproteinase (MMP) enzymes[10. Expression and activation of matrix metalloproteinases in wounds: modulation by the tripeptide-copper complex glycyl-L-histidyl-L-lysine-Cu2+. Simeon A, Monier F, Emonard H, Gillery P, Birembaut P, Hornebeck W, Maquart FX. J Invest Dermatol. 1999 Jun. 112(6):957-64.][11. The tripeptide-copper complex glycyl-L-histidyl-L-lysine-Cu2+ stimulates matrix metalloproteinase-2 expression by fiboblast cyultures. Simeon A, Emonard H, Hornebeck W, Maquart FX. Life Sci. 2000 Sep 22. 67(18):2257-65.]. This growth factor belongs to a group of emergency response molecules that come to the body’s aid in times of stress, including wound healing[12. Published studies on tissue and skin remodeling copper-peptides: copper peptide studies on skin renewal, wound healing, and hair growth. Pickart L. Skinbiology.com. 2014.], tissue remodeling,[13. The human tri-peptide GHK and tissue remodeling. Pickart L. J BiomaterSciPolym Ed. 2008. 19(8):969-88.], stem cell anti-senescence[14. Stem cell recovering effect of copper-free GHK in skin. Choi HR, Kang YA, Ryoo SJ, Shin JW, Na JI, Huh CH, Park KC. J Pept Sci. 2012 Nov. 18(11):685-90.], aging[15. Skin care benefits of copper peptide containing facial cream. Leyden J, Stephens T, Finkey MB, Appa Y, Barkovic S. Amer Academy Dermat Meeting. 2002 Feb. Abstract p68, p69.][16. The human tripeptide GHK (glycyl-L-histidyl-L-lysine), the copper switch and the treatment of the degenerative conditions of aging. Pickart L. Anti-Aging Therapeutics Vol XI. American Academy of Medicine:Chicago IL. 2009. 301-3012. Klatz R, Goldman R (eds.)], post-procedure[17. Effects of topical copper日本藤素
tripeptide complex on CO2 laser-resurfaced skin. Miller TR, Wagner JD, Baack BR, Eisbach KJ. Arch Facial Plast Surg. 2006 Jul-Aug. 8(4):252-9.][18. Ibid.], inflammation and oxidative stress[19. Effects of glycyl-histidyl-lysyl chelated Cu(II) on ferritin dependent lipid peroxidation. Miller DM, DeSilva D, Pickart L, Aust SD. Adv Exp Med Biol. 1990. 264:79-84.], and infection. The addition of copper further improves collagen synthesis and potentiates the effect of the growth factor.
Copper Tripeptide-1 also has anti-tumorigenic properties while at the same time encouraging the growth and normal development of healthy cell lines.[20. The tripeptide, GHK, induces programmed cell death in SH-SY5Y neuroblastoma cells. Matalka LE, Ford A, Unlap MT. J Biotechnol Biomater. 2012. 2:144.][21. A ‘metastasis-prone’ signature for early-stage mismatch-repair proficient sporadic colorectal cancer patients and its implications for possible therapeutics. Hong Y, Downey T, Eu KW, Koh PK, Cheah PY. Clin Exp Metastasis. 2010 Feb 9.]
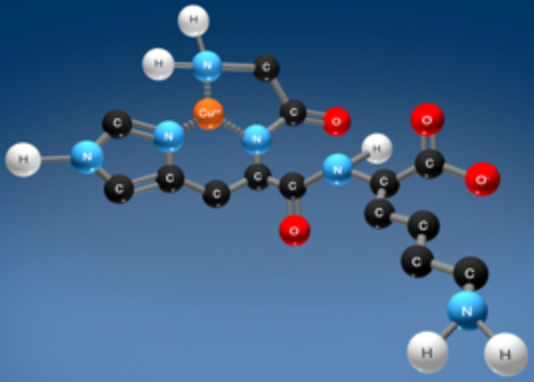
In addition, adding a second generation Vitamin C to Copper Tripeptide-1 may even further increase Collagen I synthesis.
Copper and SOD
Superoxide dismutase (SOD) is one of three enzymatic antioxidants made by the human body. This group also includes catalase and glutathione peroxidase. All were designed through evolutionary processes to neutralize free radical damage. This group of antioxidants is unique in several aspects when compared with other antioxidants such as those ingested or applied topically. The enzymatic antioxidants are effective in very tiny amounts and furthermore are not inactivated during the redox process. Furthermore, they are not used up while combating free radical processes and persist in the body for long periods of time, unlike other non-enzymatic antioxidants, such as Vitamin C. Copper can assist SOD for proper functioning in its antioxidant role[22. Superoxide dismutases – a review of the metal-associated mechanistic variations. Abreu IA, Cabelli DE. Biochim Biophys Acta. 2010 Feb. 1804(2):263-74.].
SOD, which previously required intravenous administration for delivery, is now available in topical form. Studies performed on this type of superoxide dismutase show a decrease in free damage markers with its use as well as an increase in markers of antioxidant protection.
Copper and Energy Production
Copper is involved with energy creation via its role in cytochrome oxidase. All living cells in the human body generate energy in order to function. Copper is required in mitochondria, the tiny energy factories within each cell that are responsible for metabolism and energy creation.
Copper and Melanin Synthesis
Nature designed melanin as some protection against photodamage. Both UVA and UVB rays cause free radical damage in the skin and, during daytime hours whether cloudy or sunny, our skin is bombarded with solar rays. UVA rays even penetrate glass windows. Continuing solar exposure causes visible and biochemical signs of aging skin and increases skin cancer risks. Copper is necessary for melanin synthesis within melanocytes which are found scattered along the DEJ in the basal layer of epidermis. Tyrosinase, the enzymatic partner for copper, is the most crucial enzyme required in melanin synthesis as its action is the rate limiting step in melanin production.
Proper Amounts Required
Although copper is required for human life and for many biologic processes, too much of a good thing is not good. Ingesting large amounts of copper as a supplement can be harmful and even toxic. Both copper and iron have the potential to act as pro-oxidants and increase free radical damage if found in excess.
However, providing copper in correct amounts can certainly assist many aspects of skin functionality, improve skin health, and maintain youthful vitality of skin appearance.
